The "metal" part of a nickel metal hydride battery is actually a metal intercompound. Many kinds of intermetallic compounds have been used in the manufacture of nickel-metal hydride batteries, and they fall into two main categories.
The most common are AB5, where A is A mixture of rare earth elements plus titanium (Ti); B is nickel (Ni), cobalt (Co), manganese (Mn), or aluminum (Al). The "multi-component" electrodes of some high-capacity batteries consist mainly of AB2, where A is titanium (Ti) or vanadium (V), B is zirconium (Zr) or nickel (Ni), plus some chromium (Cr), cobalt (Co), iron (Fe) and/or manganese (Mn). All of these compounds perform the same role: reversibly forming metal hydrides.
When the battery is charged, hydrogen ions (H+) are released from the potassium hydroxide (KOH) electrolyte, which are absorbed by these compounds, avoiding the formation of hydrogen (H2) to maintain the pressure and volume inside the battery. When the battery is discharged, these hydrogen ions return to their original location by the reverse process.


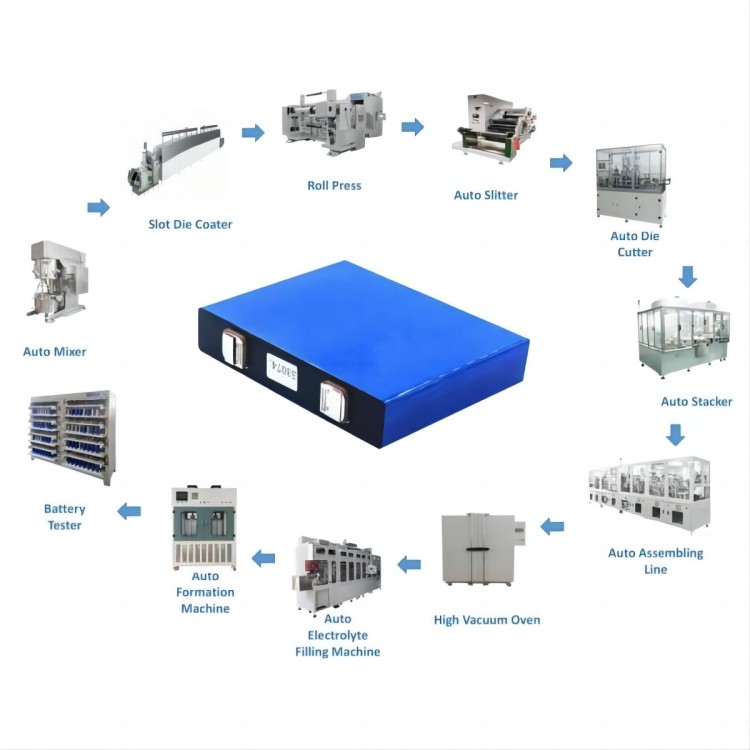
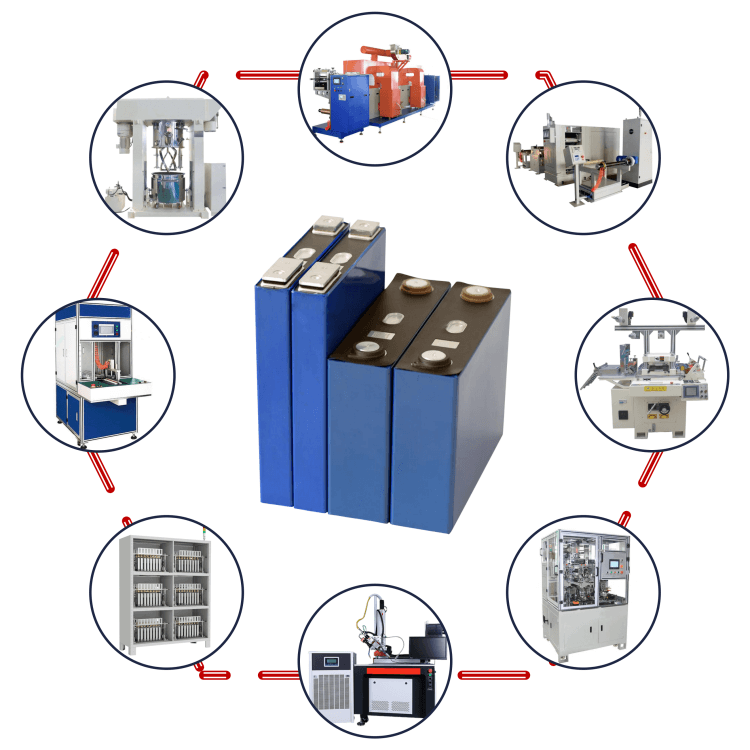
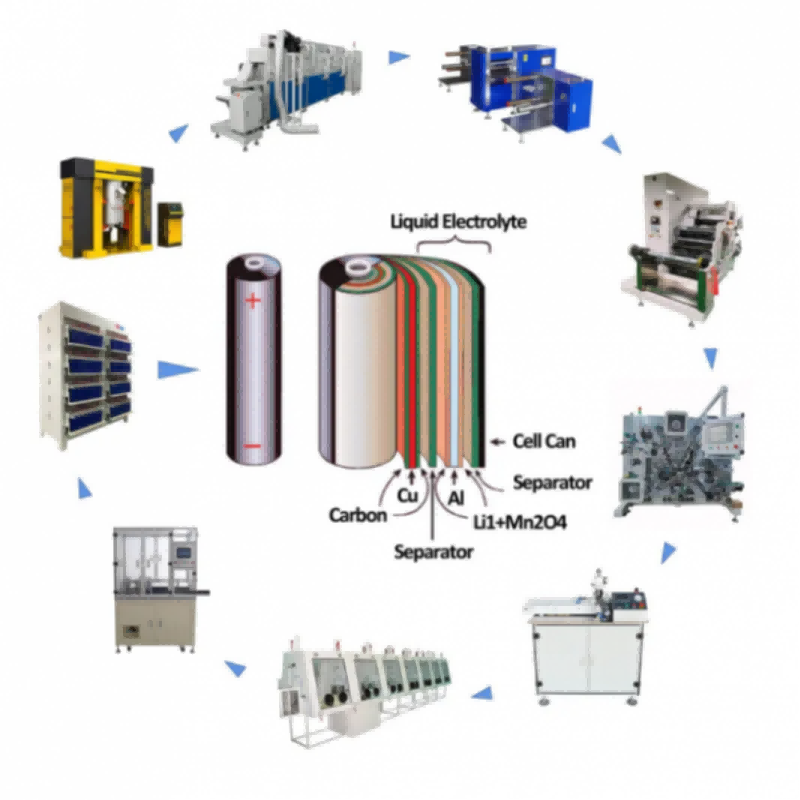

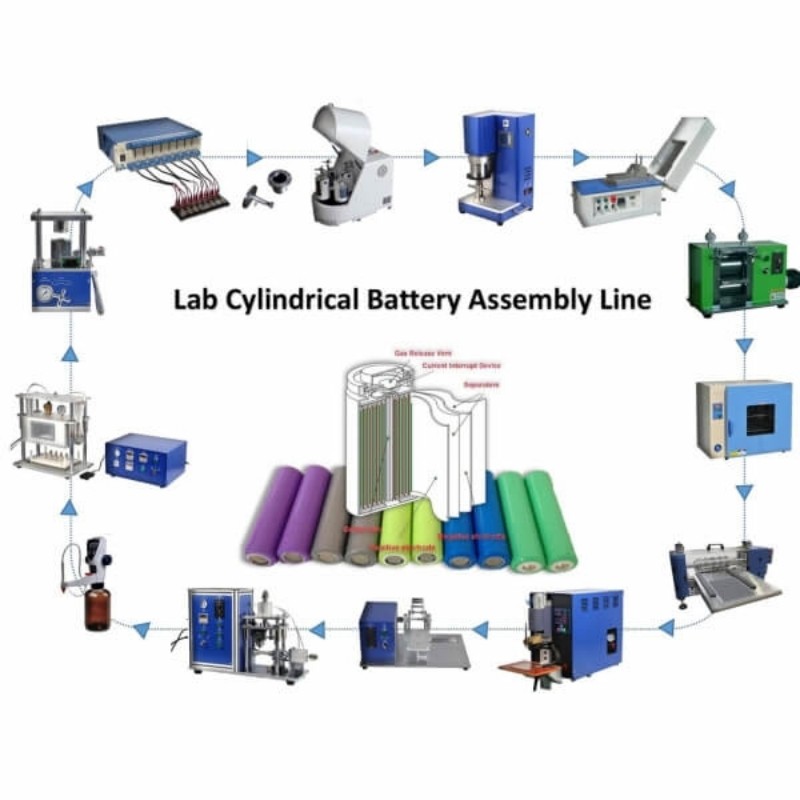


 IPv6 network supported
IPv6 network supported